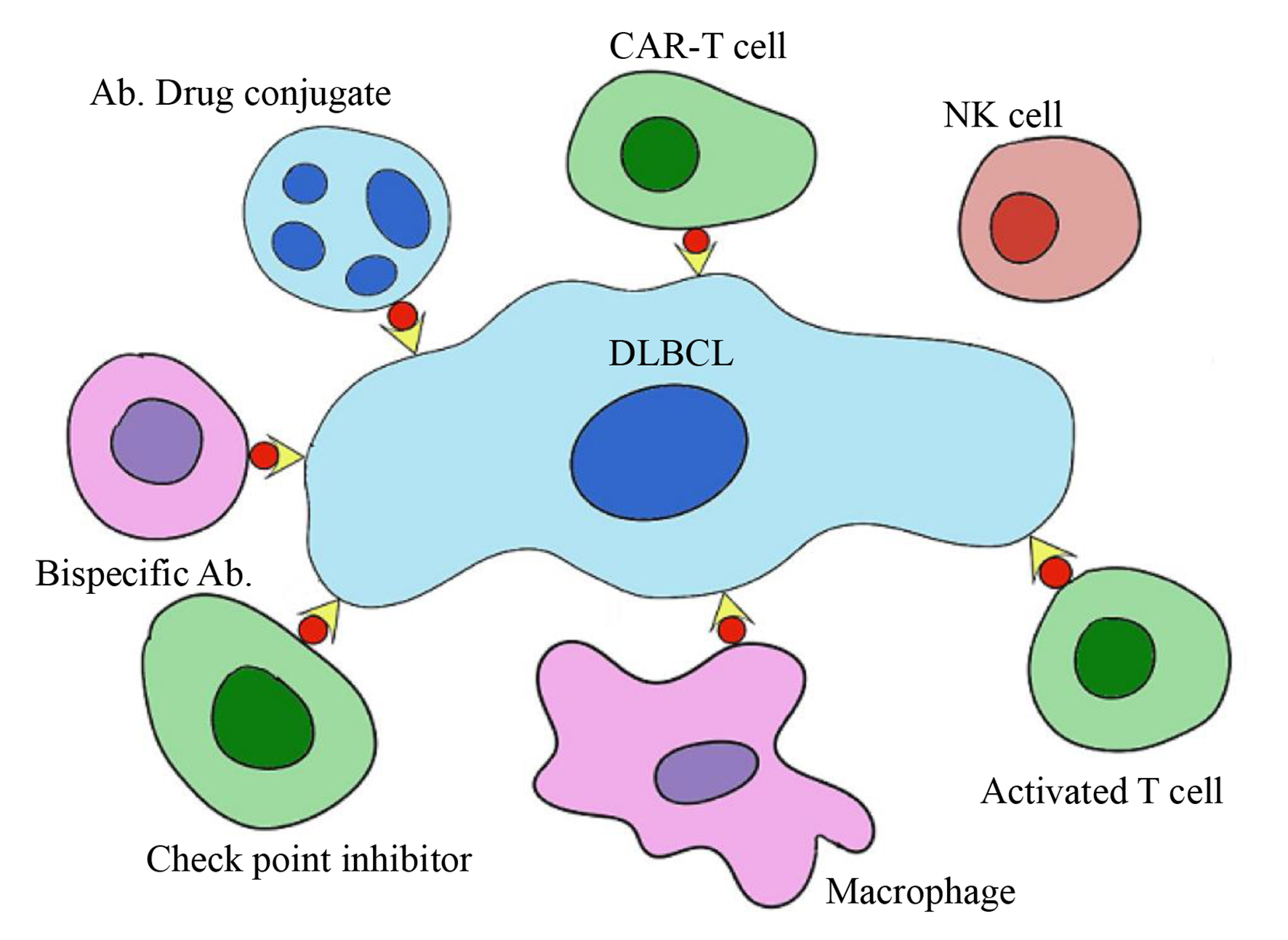
Figure 1. Immunological targets in DLBCL. DLBCL: diffuse large B-cell lymphoma.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://www.thejh.org |
Review
Volume 12, Number 4, August 2023, pages 145-160
Harnessing the Immune System: An Effective Way to Manage Diffuse Large B-Cell Lymphoma
Figures

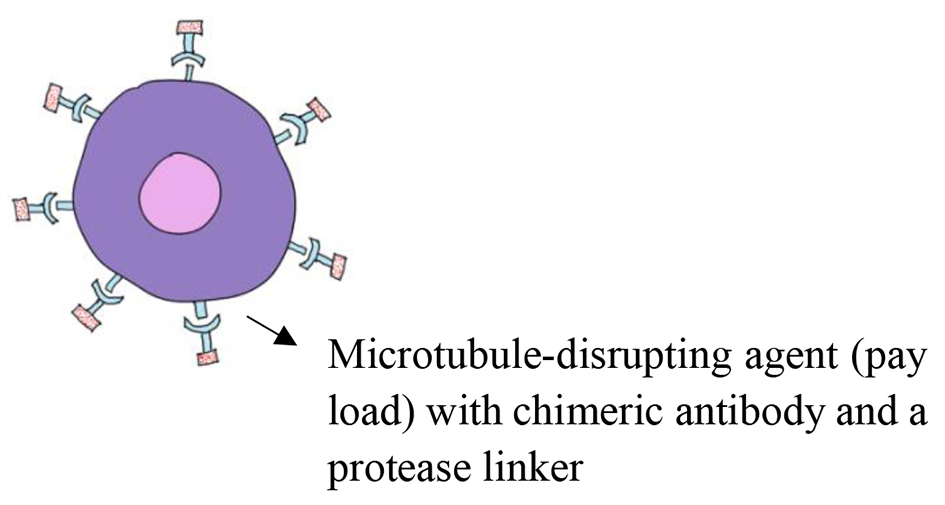
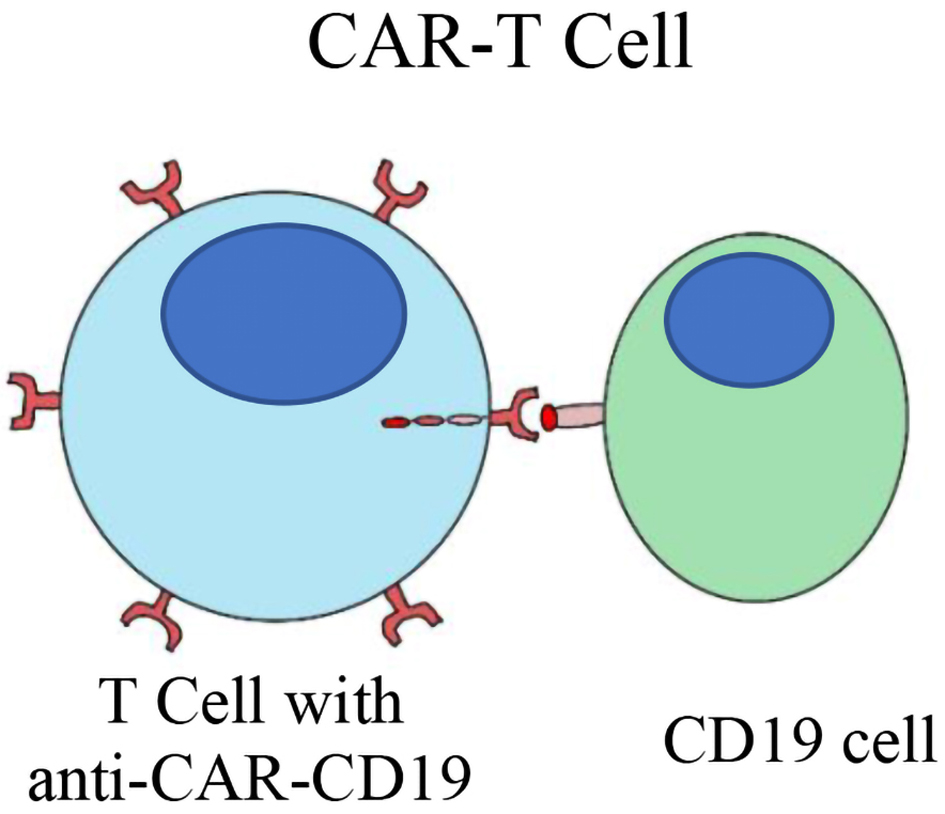
Table
| Therapeutic agent | Target | Functional characteristics | Therapeutic role in DLBCL |
|---|---|---|---|
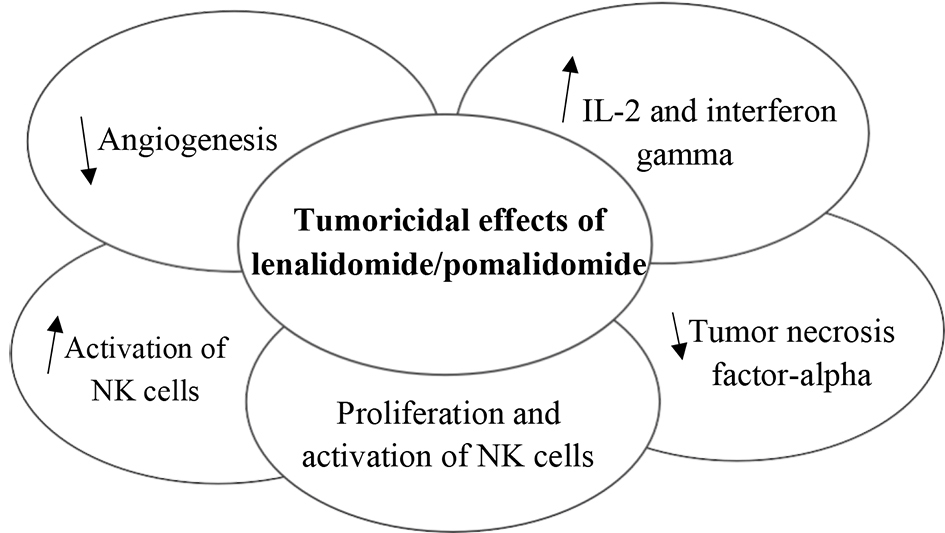
| Lenalidomide [3], pomalidomide [4] | Cereblon | LEN/POM increase NK cell function and IL2 production and decrease angiogenesis | Tafasitamab + lenalidomide in NGC DLBCL - CR 43%, PR 18%; pomalidomide and pembrolizumab in DLBCL - RR 50%, OS 14.7 months |
| Axicabtagene ciloleucel [4], tisagenlecleucel [7], lisocabtagene maraleucel [8] CAR therapy loncatuximab tesirine (ADC) [9], tafasitamab [10] (monoclonal Ab.) | CD19 | Is present during all phases of B cell development until terminal differentiation is needed for signal transduction | Tafasitamab in combination with lenalidomide is approved for treatment of R/R DLBCL patients ineligible for ASCT. Loncastuximab tesirine is approved as a third line treatment in patients with R/R DLBCL - ORR 45.6%, CR 26.7%. Axicabtagene ciloleucel, tisagenlecleucel, lisocabtagene maraleucel - axi-cel 4-year OS 43%; tisa-cel 1-year OS 55.1%; liso-cel 57.9% - there is no evidence suggestive of difference in survival between these agents. |
| Blinatumumab (BiTE) [11] | CD19, CD3 | Bispecific antibody links CD3 of effector and CD19 of tumor cells | Phase II trial in R/R DLBCL: ORR of 43% |
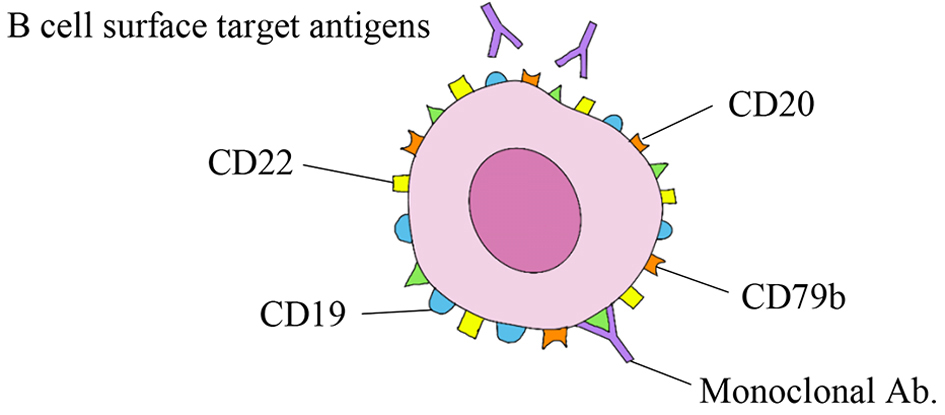
| Rituximab [5], ofatumumab [12], obinatuzumab [6] | CD20 | Needed for maturation and activation of B cells | Rituximab is approved for treatment of R/R DLBCL in various settings. Ofatumumab and obinutuzumab (ORR 37%) are not FDA approved. |
| Mosenutuzumab [13], glofitamab [14], plomatamab [15], epcoritamab [16], odonextamab [17] (BiTE) | CD20, CD3 | Bispecific T cell engager Ab. | Phase I trials in R/R DLBCL mosunetuzumab: ORR 87%; CR 71% |
| Inotuzumab ozogamycin [18] (ADC) | CD22 | Needed for B-cell receptor downstream signaling | Inotuzumab ozogamicin in R/R DLBCL: OS 9.5 months, PFS 3.7 months |
| Brentuximabvedotin [19] (ADC) | CD30 | “co-stimulatory receptor” for T cells - cytolytic effect | Is in phase I/II for R/R DLBCL with R/CHOP: ORR 44%; CR 17% |
| Polatuzumab vedotin [20] (ADC) | CD79b | ADC-designed to selectively payload | FDA approved. Based on phase II trial (in combination deliver an ultra-toxic rituximab/bendamustine in R/R DLBCL: ORR 41.5%; CR 38.7% |
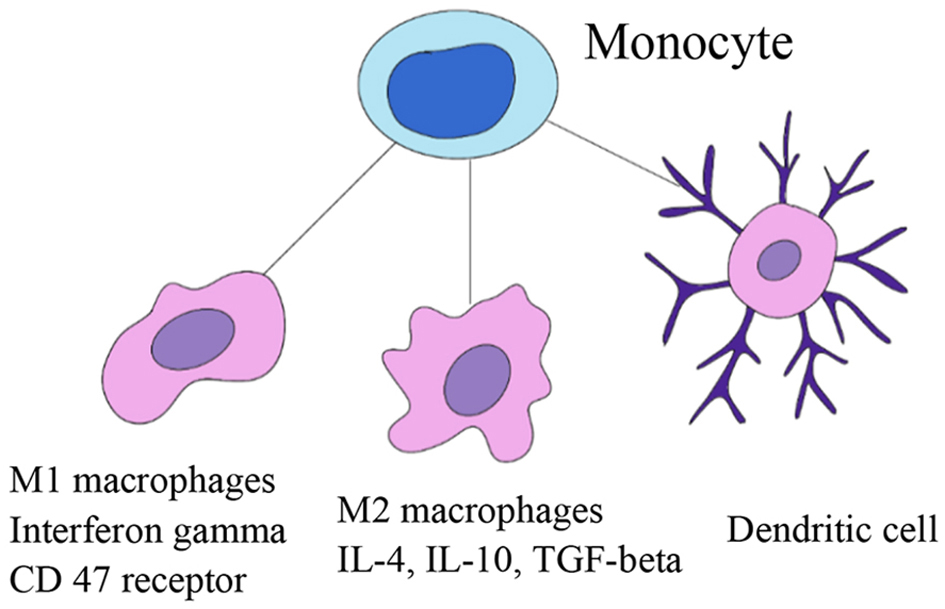
| Magrolimab [21] (monoclonal Ab.) | CD47 | Cancer cells overexpress CD47 mechanism to evade phagocytosis | TTI-622-01 (NCT03530683) is a multi-center phase 1a/1b study ongoing with R/R DLBCL. Phase 2 study: ORR 40%; CR 33% |
| Check point inhibitors [22-25] | 9p24 | Increased PDL1 expression from mutant 9p24 locus | Nivolumab - phase II study in R/R DLBCL not eligible for ABMT (ORR 10%). Pembrolizumab in phase III trial in untreated DLBCL with R-CHOP (ORR 25%). Pembrolizumab in phase Ib in R/R primary med. B cell lymphoma |